Her recall of batches of medical devices This was reported by the National Agency for Medicines., prevention of risks for fetal development in pregnant women.
The decision to recall batches of embryo culture oil was made after informing manufacturer FUJIFILM Irvine Scientific Inc. revealed the toxicity of vegetable oil which may result in damage to the development of the embryo and underdevelopment of the blastocyst or an intolerant blastocyst, making it impossible to perform the procedure with a weakened embryo.
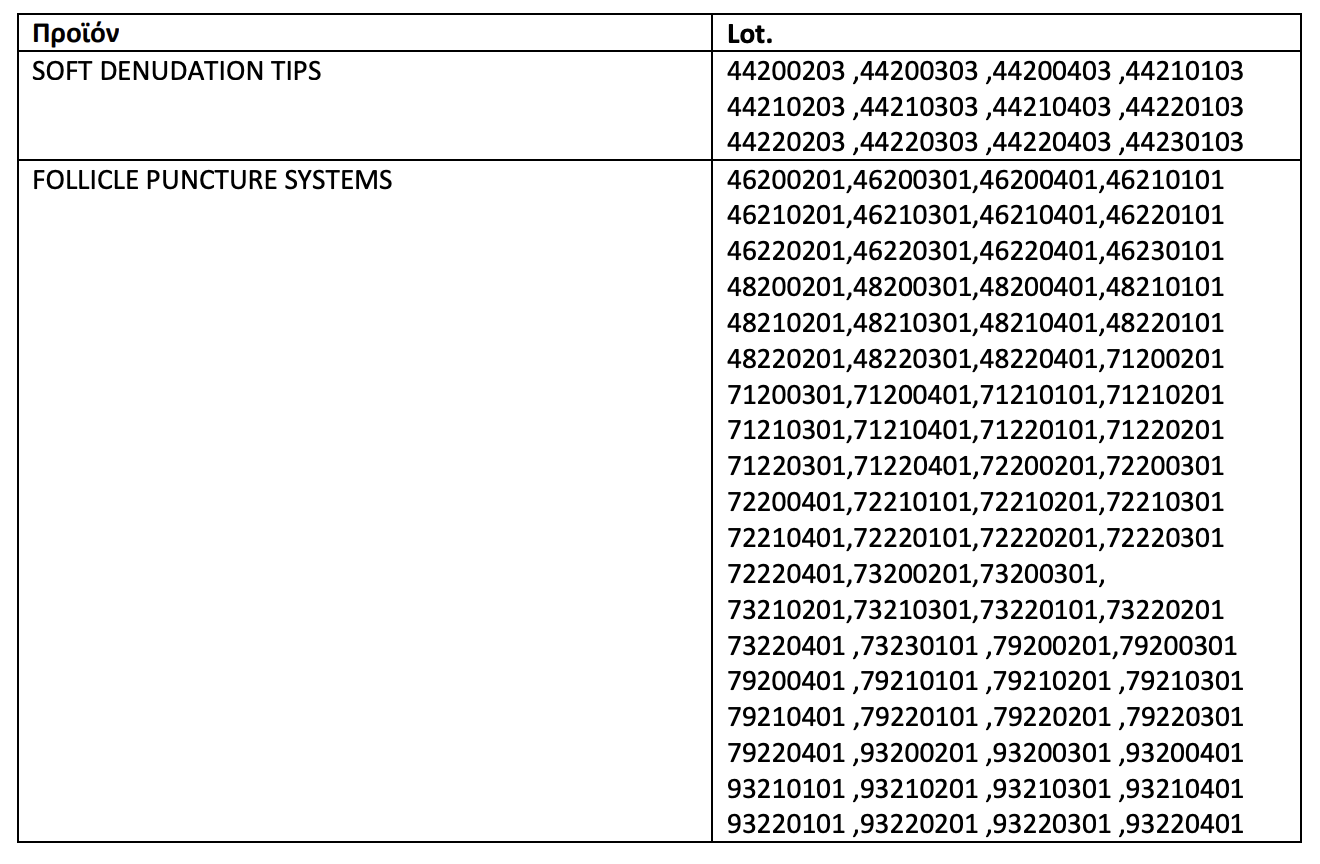
The review applies to the following games
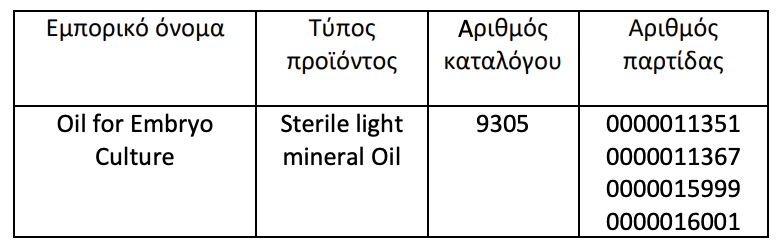
Source: Kathimerini
Ashley Bailey is a talented author and journalist known for her writing on trending topics. Currently working at 247 news reel, she brings readers fresh perspectives on current issues. With her well-researched and thought-provoking articles, she captures the zeitgeist and stays ahead of the latest trends. Ashley’s writing is a must-read for anyone interested in staying up-to-date with the latest developments.