
EOF abolishes the circulation of injectable drugs.
The decision to recall, EOF said in a statement, was made because “after receiving information about the possibility of detecting stainless steel particles in a sterile solution that come from a component that is part of the product’s production line.”
These are batches of the following two drugs:
• CELESTONE CHRONODOSE INJ.SU.RET (3+3)MG/1 ML BOTTLE
• PROPIOCHRON ENG.SUSP (5+2) mg/1ml
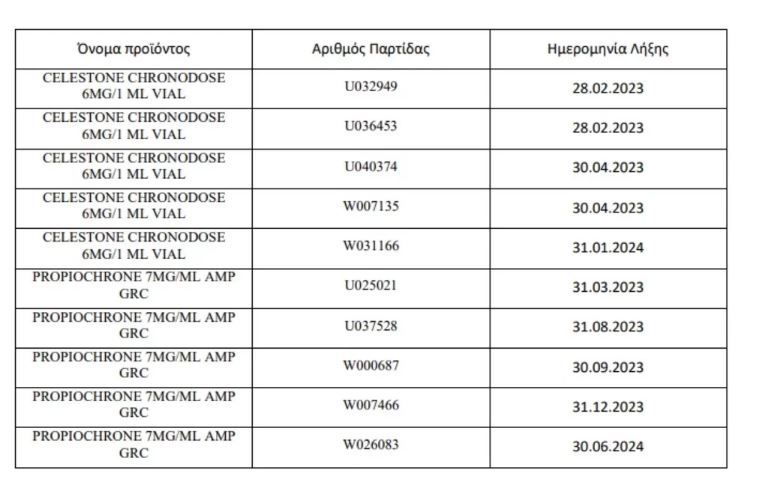
Details of recalled drugs, lot number and expiration date for each:
The active substance of both drugs is betamethasone, which, according to its activity, belongs to potent local corticosteroids and has an anti-inflammatory effect.
They are indicated in the treatment of severe and moderate diseases, in acute and chronic self-limiting diseases, amenable to systemic treatment with corticosteroids.
The EOF decision was taken as a precautionary measure in the context of protecting public health, the report says.
Tables with batches of recalled drugs:
Source: Kathimerini
Ashley Bailey is a talented author and journalist known for her writing on trending topics. Currently working at 247 news reel, she brings readers fresh perspectives on current issues. With her well-researched and thought-provoking articles, she captures the zeitgeist and stays ahead of the latest trends. Ashley’s writing is a must-read for anyone interested in staying up-to-date with the latest developments.